Abstract
Early detection of atrial fibrillation (AFib) is crucial for altering its natural progression and complication profile. Traditional demographic and lifestyle factors often fail as predictors of AFib. This study investigated pre-operative, circulating microRNAs (miRNAs) as potential biomarkers for post-operative AFib (POAF) in patients undergoing coronary artery bypass grafting (CABG). We used an array polymerase chain reaction method to detect pre-operative, circulating miRNAs in seven patients who subsequently developed POAF after CABG (cases) and eight patients who did not develop POAF after CABG (controls). The top 10 miRNAs from 84 candidates were selected and assessed for their performance in predicting POAF using machine learning models, including Random Forest, K-Nearest Neighbors (KNN), XGBoost, and Support Vector Machine (SVM). The Random Forest and XGBoost models showed superior predictive performance, with test area under the curve (AUC) values of 0.76 and 0.83, respectively. Differential expression analysis revealed four upregulated miRNAs—hsa-miR-96-5p, hsa-miR-184, hsa-miR-17-3p, and hsa-miR-200-3p—that overlapped with the POAF-miRNA signature. The POAF-miRNA signature was significantly associated with various cardiovascular diseases, including acute myocardial infarction, hypertrophic cardiomyopathy, and heart failure. Biological pathway analysis indicated these miRNAs target key signaling pathways involved in cardiovascular pathology, such as the MAPK, PI3K-Akt, and TGF-beta signaling pathways. The identified miRNAs demonstrate significant potential as predictive biomarkers for AFib post-CABG, implicating critical cardiovascular pathways and highlighting their role in POAF development and progression. These findings suggest that miRNA signatures could enhance predictive accuracy for POAF, offering a novel, noninvasive approach to early detection and personalized management of this condition.
Similar content being viewed by others
Introduction
Post-operative atrial fibrillation (POAF) represents a prevalent complication following coronary surgery, bearing a twofold increase in cardiovascular mortality and morbidity compared to individuals maintaining normal sinus rhythm1. POAF manifests in paroxysmal, persistent, or permanent forms, with onset frequently occurring within the initial 5 days, peaking at 48 to 72 h post-surgery2. Incidence rates, estimated to be between 10 and 50%, underscore its clinical significance3,4. Importantly, POAF is linked to severe adverse outcomes, including an elevated risk of venous thromboembolism (VTE) and stroke due to hemodynamic instability, impaired ventricular filling, and compromised cardiac output. Moreover, POAF may contribute to long-term cardiac pathology, exacerbating heart failure and culminating in renal decline, increased mortality, and augmented healthcare costs associated with extended hospital stays and emergent adverse events3.
In the context of coronary artery bypass grafting (CABG), POAF is not exclusive to the choice of on- or off-pump approaches and is hypothesized to result from reperfusion injury in susceptible individuals with predisposing risk profiles. Risk indices have been devised to stratify patients based on their individual risk profiles3. The intricate array of underlying factors contributing to POAF includes pre-existing cardiac and vascular pathologies, inflammatory and oxidative stress, genetic predisposition, and intra/post-operative factors triggering compensatory cardiac remodeling2. Vulnerable populations, such as those with congestive heart failure, mitral valve pathology, advanced age, or atrial interstitial fibrosis, are particularly prone to POAF development5. Patients with coronary heart disease, hypertension, and ventricular hypertrophy are at risk for permanent atrial fibrillation (AFib)6.
Despite extensive research, the mechanisms leading to POAF remain inadequately understood. Animal models, in vitro studies, and clinical trials indicate oxidative injury, inflammatory processes, altered myofibrillar energetics, neurohormonal activation, and volume overload as potential contributing factors7,8,9. Additionally, autoantibodies targeting M2 muscarinic receptors, myosin, or heat shock proteins, and chronic infections like chlamydial and Helicobacter pylori have been proposed as contributors, although causality remains to be established10,11. The relationship between AFib development independent of surgical exposure and the mechanisms involved in POAF remains unclear. Despite the development of risk indices based on clinical parameters, predictive biomarkers remain elusive. Current preventive measures, although diverse, have not substantially reduced POAF incidence following CABG. Recently, microRNAs (miRNA) have emerged as promising biomarkers, offering potential for predicting disease onset and outcomes12,13. These stable, non-coding RNAs play a crucial role in gene expression regulation across various biochemical pathways14. A seminal study by Barth et al. in 2005 observed distinct gene expression patterns in patients with permanent AFib compared to those in sinus rhythm among patients undergoing open heart surgery for valve repair or CABG, suggesting the potential of miRNAs as indicators of POAF risk15.
The stable expression of circulating miRNAs post-sample collection is a characteristic that suggests these molecules have the potential to serve as reliable biomarkers for predicting future disease events, e.g., POAF. Alterations in miRNA levels are intricately linked to the signaling cascades governing electrical remodeling and atrial fibrosis in the heart. Therefore, specific miRNAs and their quantitative profiles potentially could serve as indicative markers for a spectrum of clinical scenarios related to the heart, e.g., acting as either favorable or unfavorable indicators for the onset of AFib16,17. Evidence indicates that miRNAs serve as predictive markers for AFib in patients with cardiovascular diseases. For instance, Galenko et al.18 identified circulating miRNAs capable of discerning individuals at a heightened risk of AFib, and observed an association between reduced expression of miRNA-21 and AFib. Kiliszek et al.19 also illustrated significant differences in the levels of 34 miRNAs in sera from patients with AFib recurrence compared to those without AFib recurrence. Additionally, Cao et al.20 identified seven upregulated and 13 downregulated differentially expressed miRNAs to distinguish patients with AFib from healthy individuals. However, given the complexities associated with AFib, there remains a need to explore feasible biomarkers that can predict first occurrence of AFib to prevent adverse outcomes in patients with cardiovascular diseases.
This study aimed to investigate whether miRNAs can predict POAF following CABG. We used machine learning methods to identify a group of circulating miRNAs, measured pre-operatively, that could distinguish between patients who went on to develop POAF and those who maintained sinus rhythm following CABG. We also determined whether this group of miRNAs had known associations with cardiovascular diseases, tested for enrichment of these miRNAs in biological pathways, with an emphasis on enrichment in pathways related to cardiovascular disease, and identified the target genes of these miRNAs to form a miRNA-target gene interaction network. The results will provide insights into the target genes and biologically relevant pathways that are impacted during the emergence of POAF.
Methods
Study subject inclusion and exclusion criteria
The Internal Review Board (IRB) from Marshfield Clinic Health System approved this cohort study prior to data access and determined that the study posed minimal risk to participants. All the data extraction methods were carried out in accordance with the IRB guidelines and regulations.
Patients who were candidates for CABG at Marshfield Clinic Health System (MCHS) were identified by a MCHS cardiologist (JB) as he encountered them in his practice, by reviewing the scheduled surgeries of three MCHS cardiologists who performed CABG procedures, or by alerts from medical staff of emergent, unscheduled CABG procedures to be performed by the three MCHS cardiologists. Before the CABG procedure was performed, identified patients were screened for study eligibility by a review of electronic health record (EHR) data conducted by study staff. Inclusion criteria were age ≥ 18 years at time of screening, patient was an established MCHS patient, CABG surgery was to be performed at MCHS, and patient had no history of a prior CABG procedure. Exclusion criteria were patient had a previous history of AFib, patient had a previous CABG performed, patient was in shock, patient had a previous heart valve surgery, patient had a myocardial infarction with balloon pump procedure within 72 h prior to CABG, patient was scheduled for cardiac procedures in addition to CABG, patient received a blood transfusion within two weeks prior to CABG, patient had an infection requiring antibiotics, patient was unable to communicate effectively enough to report POAF symptoms following discharge, patient had a history of non-compliance, and patient or caregiver had personal or cultural beliefs regarding the patient’s condition or medical care which made it unlikely they would report symptoms of AFib after hospital discharge, potentially producing misclassification error among controls. A MCHS cardiologist (JB) performed a second review of the EHR data to verify that patients met study eligibility criteria.
Participant recruitment
Following screening and before the CABG procedure, eligible patients were provided with a description of the study and asked to provide informed consent to participate in the study. Informed consent was obtained from all subjects and/or their legal guardian(s). A short interview was conducted with participants who provided informed consent to obtain information not readily available in the EHR, including history of blood transfusions, use of prescription and over-the-counter medications, previous surgeries, previous cardiac-related hospitalizations, family history of cardiac and other serious diseases, and consumption of caffeine, alcohol, and tobacco products. Study participants also provided a 10 mL blood sample prior to their CABG procedure.
Participant follow-up and ascertainment of case–control status
Within 30 days following patient discharge after the CABG procedure, details of the CABG procedure were extracted from the EHR by MCHS cardiologists. The details included type of CABG surgery (on- or off-pump), time on pump, number of vena cannulas used, type of anesthesia, length of surgery, aortic clamp time, type and amount of blood products received (if any), lowest hematocrit, lowest body (bladder) temperature achieved, perioperative medications, diagnosis of AFib following CABG, and occurrence of infection or stroke while hospitalized.
Cases were defined as patients who developed POAF within 30 days after the CABG procedure. Controls were patients who remained in sinus rhythm for 60 days following the CABG procedure. A MCHS cardiologist contacted patients by telephone once 30 days had elapsed following the CABG procedure to ask the patients whether they had been diagnosed with POAF following the procedure. For patients who reported POAF, the cardiologist confirmed the diagnosis by reviewing EHR data or, if the patient was treated at another facility after CABG surgery, obtaining and reviewing medical records from other facilities. For patients without POAF, the telephone interview also determined if, within the 30-day period, patients had an acute cerebral vascular event, received blood products, had other cardiac procedures, felt fluttering or pain in the chest, or experienced dizziness. At 60 days after the CABG procedure, patients were interviewed by telephone again to inquire about their health status. The patients diagnosed with POAF within 30-days after CABG were asked whether they had an infection that required treatment with an antibiotic, an acute cerebral vascular event, had received any blood products, or had any cardiac procedures, and, if yes, whether these occurred before or after the POAF diagnosis. The patients who did not have POAF within 30-days after CABG were asked whether they had an acute cerebral vascular event, received blood products, had other cardiac procedures, felt fluttering or pain in the chest, or experienced dizziness in the 30–60 day period after the CABG procedure. Patient EHR data were reviewed by MCHS cardiologists to verify patient reports given in the telephone interviews, and the clinical data were used to categorize patients by case–control status.
Processing of blood samples, extraction of miRNA, and measurement of miRNA expression
Blood samples were collected in 10 mL tubes that contained no anticoagulants. The blood was allowed to clot in the tubes for 1–2 h at room temperature before centrifugation (3000 rpm for 10 min) at 4 °C. miRNA was extracted from the serum collected after centrifugation using the miRNeasy Serum/Plasma Kit (Qiagen, Valencia, CA), according to the manufacturer’s instructions. The quality (A260:A280 ratio) of the extracted miRNA was measured using a NanoDrop™ spectrophotometer (ThermoFisher Scientific, Waltham, MA). The expression of a panel of 84 miRNAs was measured by reverse transcription quantitative polymerase chain reaction (qPCR) in the miRNA samples using the Human Serum & Plasma miScript miRNA PCR Array (Qiagen), following the manufacturer’s instructions, on a LightCycler 480 Instrument (Roche, Indianapolis, IN). The 84 miRNAs were selected by the manufacturer for inclusion on the panel because the serum expression levels of the miRNAs had been correlated with heart disease, liver disease, atherosclerosis, diabetes, and certain cancers in previous research. Delta cycling threshold (∆Ct) values for each miRNA were calculated by subtracting the average Ct value for six housekeeping small RNAs (SNORD61, SNORD68, SNORD72, SNORD95, SNORD96A, and RNU6-2) from the Ct value for the miRNA.
Statistical analysis
Descriptive statistics are presented as medians and interquartile ranges (IQRs) for continuous variables, and as counts and percentages for categorical variables. Baseline characteristics were compared using the Wilcoxon test for continuous data and the Pearson \(\chi^{2}\) test for categorical data. All statistical analyses were conducted using R version 4.3.1 (R Project for Statistical Computing) with the tidycmprsk and ggplot2 packages.
Machine learning methods
We utilized standard machine learning algorithms to differentiate between POAF and control groups. The dataset was divided into 80% training (n = 12) and 20% validation (n = 3) subsets. A correlation-based feature selection method was applied to identify the top 10 miRNAs out of 84, with a correlation threshold set at 0.7. Random Forest, eXtreme Gradient Boosting (XGBoost), K-Nearest Neighbors (KNN), and Support Vector Machine (SVM) methods were employed for classification. The models were built using R libraries including ‘randomForest’, ‘xgboost’, ‘kNN’, and ‘caret’. The prediction performance of the models was evaluated using receiver operating characteristic (ROC) curves with the R package ‘pROC’.
The correlation coefficient between two miRNAs, A and B can be calculated using the following equation.
where Ai and Bi are the individual data points for miRNAs A and B respectively.
\(\overline{A}\) and \(\overline{B}\) are the means of miRNAs A and B respectively.
Differential expression analysis
In this study, we analyzed miRNA expression profiles from patients with POAF and control subjects using DESeq2. DESeq2’s statistical framework, based on a negative binomial distribution, accounts for variance-mean dependence and the biological variability typical of RNA-seq experiments. Although we have ΔCT values from PCR experiments, we opted to use DESeq2 for the statistical analysis. DESeq2 calculated normalized expression values, applied shrinkage estimators for variance stabilization, and conducted Wald tests to determine fold changes and associated p-values. To minimize false positives, a false discovery rate (FDR) adjusted q-value threshold of 0.05 was used.
MiRNA-disease association and pathway analysis
We utilized miRNA-disease association information from HMDD v4.021 and miRNet22. The POAF-miRNA signature and its disease associations were visualized using bar plots created with the ggplot2 (ver. 3.4.3) R package. The POAF-miRNA signature and its links to cardiovascular-specific diseases were displayed using an alluvial plot generated with the Alluvial R package. Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analysis was conducted with KEGG database23 and DIANA-miRPath v4.024. For Gene Ontology (GO) categories and Reactome pathways, we used both DIANA-miRPath v4.024 and miRNet22. Specific pathways were visualized as bar plots using ggplot2 (ver. 3.4.3), and cardiovascular-specific pathways were displayed using chord diagrams created with the circlize R package.
MiRNA-gene target interaction
To identify gene targets for each miRNA, we employed four different miRNA-gene target databases: miRWalk25, miRNet22, miRDB26, and miRTarBase27. We ensured robust predictions by including only gene targets found in at least two of these databases. To further refine our results, we focused on gene targets supported by all four databases, ultimately identifying 50 genes associated with the POAF-miRNA signature. We used the ComplexUpset (version 1.3.3) R package to create upset plots.
Results
Patient characteristics
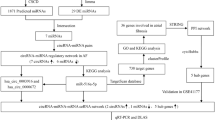
A total of 164 patients were screened for study eligibility. After applying the inclusion and exclusion criteria, 84 subjects were excluded due to various reasons, such as having a prior diagnosis of atrial fibrillation (n = 26), CABG (coronary artery bypass grafting) surgery performed previously or scheduled at another facility (n = 15 and n = 2, respectively), or other disqualifying factors as outlined in the flowchart (Fig. 1).
Of the remaining 80 subjects, 64 were further excluded due to declining to participate (n = 18), not completing the consent process before surgery (n = 17), or not being confirmed as eligible (n = 13). Ultimately, 16 subjects (8 cases and 8 controls) provided informed consent and were enrolled in the study. One case was excluded post-enrollment due to the development of atrial flutter following CABG surgery, resulting in a final cohort of 15 subjects (7 cases and 8 controls) for the miRNA analysis.
Baseline characteristics
The baseline characteristics of the study cohort are detailed in Table 1. The mean age at CABG surgery was 71.4 ± 7.7 years for cases and 68.4 ± 7.7 years for controls. Both groups consisted predominantly of male (cases: 85.7%, controls: 87.5%) and non-Hispanic white individuals (100% in both groups). The majority of participants in both groups were married (cases: 71.4%, controls: 75.0%).
Important clinical features included a high prevalence of alcohol consumption among cases (57.1%) compared to controls (25.0%). Tobacco use was observed in 14.3% of cases versus 25.0% of controls. All cases (100%) and 87.5% of controls had used aspirin daily. Further clinical history characteristics and other pertinent variables are detailed in Table 1.
To address comorbidities as potential confounders, we observed that hypertension affected 71.4% of cases and 87.5% of controls, while hyperlipidemia was present in 85.7% of cases and 87.5% of controls. Cases exhibited unique comorbidities, including abdominal aortic aneurysm (14.3%), seizures (14.3%), stroke (14.3%), and deep vein thrombosis (14.3%), the first of which (POAF) was already controlled for in the original analysis. Controls showed a higher prevalence of obstructive sleep apnea (25.0%), chronic obstructive pulmonary disease (25.0%), chronic kidney disease (12.5%), heart failure with preserved ejection fraction (12.5%), morbid obesity (12.5%), and congestive heart failure (12.5%), which were absent in cases (Supplementary Table S1).
Medication use among the cohort (7 cases, 8 controls) reflected the cardiovascular focus of the study, with a total of 101 medication instances across 15 participants (Table 4). The most prevalent categories were vasodilators/nitrates (17 instances, used by 73.3% of participants), statins (13 instances, 66.7%), beta-blockers (7 instances, 40.0%), calcium channel blockers (6 instances, 40.0%), and ACE inhibitors (6 instances, 40.0%). Beta-blocker use was balanced between cases (42.9%) and controls (37.5%), with no significant difference (p = 0.832). Similarly, the other top medication categories showed no significant differences in usage between groups (p-values ranging from 0.293 to 0.832). Notably, no participants were prescribed anticoagulant medications (e.g., warfarin, heparin, or direct oral anticoagulants), despite the cardiovascular context, which may reflect specific clinical management strategies in this cohort. Polypharmacy was evident, with each participant receiving 2 to 12 medications, reflecting the complexity of managing post-CABG patients with multiple comorbidities (Supplementary Table S2).
Statistical analysis
Both cases and controls had similar mean ages with overlapping standard deviations. The t-test revealed no significant (p ≥ 0.05) age difference between the groups.
A chi-square analysis was conducted to assess the association between POAF and two categorical factors: alcohol consumption and tobacco use. The results indicated that alcohol consumption was not significantly associated with POAF, as the p-value was 0.46. Similarly, no significant relationship was observed between tobacco use and POAF, with a p-value of 1.0. The results suggested that none of these factors were significantly associated with POAF in this cohort. However, due to the limited sample size, further investigation with larger patient groups is warranted to validate these findings.
Identifying the miRNAs predictive of POAF
To identify the miRNAs that are predictive of POAF, we employed a correlation-based feature selection method. We eliminated the features that were highly correlated (R < 0.80) and selected the top 10 ranked miRNAs out the 84 miRNAs. We divided the dataset into training and validation sets (at a 7:3 ratio) and evaluated the performance of four machine learning methods—Random Forest, K-Nearest Neighbor (KNN), XGBoost, and Support Vector Machine (SVM)—in classifying POAF and control samples using the 10 selected miRNAs obtained from correlation-based feature selection. These 10 miRNAs, including hsa-miR-19a-3p, hsa-miR-19b-3p, hsa-miR-184, hsa-miR-200a-3p, hsa-let-7a-5p, hsa-miR-124-3p, hsa-miR-423-5p, hsa-miR-96-5p, hsa-miR-100-5p, and hsa-miR-17-5p, were collectively termed as an POAF-miRNA signature. The Random Forest model demonstrated perfect performance on the training dataset with an accuracy, sensitivity, specificity, and AUC of 1.0. However, its performance slightly dropped on the test dataset, with an accuracy, sensitivity, specificity, and an AUC of 0.80, 0.87, 0.71, and 0.76, respectively. The KNN model showed a balanced performance across both datasets. On the training dataset, it achieved an accuracy, sensitivity, specificity, and an AUC of 0.75, 0.62, 1.0, and 0.84, respectively. On the test dataset, it achieved an accuracy of 0.80, sensitivity of 0.62, specificity of 1.0, and an AUC of 0.77. XGBoost yielded high performance on both datasets. It achieved an accuracy, sensitivity, specificity, and an AUC of 0.9, 0.90, 0.89, and 0.97, respectively, on the training dataset. On the test dataset, it maintained a good accuracy, sensitivity, specificity, and an AUC of 0.73, 0.75, 0.71, and 0.83, respectively. The SVM model had the lowest performance among the evaluated methods, and obtained an accuracy, sensitivity, specificity, and an AUC of 0.55, 0.62, 0.46, and 0.53, respectively on the training dataset. Its performance further declined on the test dataset, as shown in Table 2.
Overall, XGBoost exhibited the highest AUC on both training and test datasets, indicating its superior performance in distinguishing POAF from control samples. XGBoost outperformed other machine learning models, likely due to its ability to handle high-dimensional data with a small sample size by applying regularization and leveraging iterative boosting to optimize classification performance28,29. The model’s ability to capture non-linear relationships between miRNA expression and POAF risk makes it well-suited for biomarker-based disease prediction. Our findings align with prior research demonstrating that ensemble-based models, such as XGBoost and Random Forest, often achieve higher predictive accuracy in biological and clinical datasets compared to traditional linear classifiers.
These results underscore the importance of model selection and validation in the development of robust classifiers for POAF detection using miRNAs. The prediction performance of these models were evaluated using ROC curves, as shown in Fig. 2. To evaluate the predictive relevance of individual miRNAs, we employed XGBoost-based feature importance ranking, which quantifies how often a feature contributes to model decision splits and classification performance. The top-ranked miRNAs—hsa-miR-200a-3p, hsa-let-7a-5p, and hsa-miR-423-5p—exhibited the highest importance scores, suggesting their strong association with POAF risk. (Supplementary Fig. S1) To complement this, we applied Shapley Additive Explanations30 analysis to further elucidate the influence of each miRNA across individual predictions. The results confirm that a subset of miRNAs contributes more prominently to classification, while others may have limited predictive value in this dataset.
We performed external validation of the panel of 10 miRNAs using the GSE22273931 dataset from the Gene Expression Omnibus (GEO). This dataset had plasma miRNA expression profiles from epicardial adipose tissue from the right atrium of 4 POAF cases and 5 controls (without POAF) who had undergone CABG surgery. Our study had used serum miRNA data to identify the panel of 10 miRNAs but no serum miRNA data were available in the GSE222739 dataset. Challenges for the validation analysis included differences between heart tissue and serum miRNA compositions, which can affect model performance, and the small size of the validation dataset, likely leading to overfitting and variability. Additionally, since the GSE222739 dataset lacked data for one of the 10 miRNAs, we imputed the expression values hsa-let-7a-5p.
Despite these issues, Leave-one-out cross-validation was used, with Random Forest and KNN models achieving 67% accuracy for the panel of 10 miRNAs in GSE222739. When the miRNAs were considered individually, five of the miRNAs, e.g., hsa-miR-19b-3p and hsa-miR-124-3p, showed up to 56% accuracy, suggesting their potential as biomarkers, but others like hsa-miR-184 had poor accuracy, indicating context-dependency (Supplementary Table S3).
Identification of differentially expressed miRNAs
Differential expression analysis (DEA) was employed to evaluate expression variations in the miRNA expression profiles of patients with POAF (n = 7) and controls (n = 8). Differentially expressed miRNAs were screened based on fold change and a FDR threshold of q < 0.05. A total of 13 miRNAs were identified, with 12 being upregulated, including hsa-miR-96-5p, hsa-miR-184, hsa-miR-208a-3p, hsa-miR-17-5p, hsa-miR-499a-5p, hsa-miR-200a-3p, hsa-miR-203a, hsa-miR-210-3p, hsa-miR-9-5p, hsa-miR-204-5p, hsa-miR-146a-5p, and hsa-miR-133b. One miRNA, hsa-miR-574-3p, was downregulated, as shown in Fig. 3. Four of these miRNAs—hsa-miR-96-5p, hsa-miR-184, hsa-miR-17-3p, and hsa-miR-200a-3p—overlap with the POAF-miRNA signature.
POAF-miRNAs and disease association
The POAF-miRNAs target various genes that can contribute to the development of disease pathways. These miRNAs are involved in numerous disease pathways, including cardiovascular diseases, cancers, and other non-cardiovascular diseases. The top 10 significant (p < 0.001) non-cardiovascular diseases associated with these miRNAs include vulvar carcinoma, multiple sclerosis, retinoblastoma, kaposi’s sarcoma, medulloblastoma, chronic kidney disease, macular degeneration, B-Cell leukemia, Crohn’s disease, and myeloid leukemia. miRNAs such as hsa-let-7a-5p, hsa-miR-96-5p, hsa-miR-124-5p, and hsa-miR-17-5p showed enrichment in multiple diseases (Supplementary Fig. S2), suggesting their broader impact on disease pathology. The majority of the diseases associated with these miRNAs are related to cancer.
Furthermore, we identified associations between seven miRNAs in the POAF-miRNA signature and cardiovascular diseases. The miRNAs hsa-miR-124-3p, hsa-miR-17-5p, hsa-miR-184, hsa-miR-19a-3p, hsa-miR-19b-3p, hsa-miR-423-5p, and hsa-miR-96-5p showed statistically significant (p < 0.005) associations with POAF, acute myocardial infarction, hyperactivity disorder, hypertrophic cardiomyopathy, pulmonary hypertension, and vascular disease, as shown in Fig. 4.
Biological relevance of the POAF-miRNA signature
We investigated the biological relevance of the miRNAs using KEGG, GO, Reactome pathway analysis. The POAF-miRNA signature plays crucial roles in various biological functions, impacting multiple diseases, including cardiovascular diseases. In KEGG pathway analysis, the POAF-miRNA signature was significantly enriched in pathways such as proteoglycans in cancer, p53 signaling pathway, hepatitis B, EGFR tyrosine kinase inhibitor resistance, and AGE-RAGE signaling pathway in diabetic complications, as detailed in Supplementary Table S4. The enrichment analysis of the POAF-miRNA signature is illustrated in Supplementary Fig. S3A. Focusing on POAF-miRNA signature enriched in cardiovascular-related pathways, we identified specific KEGG pathways involved in cardiovascular diseases, including hypertrophic cardiomyopathy, MAPK signaling, PI3K-Akt signaling, FoxO signaling, and TGF-beta signaling (Table 3 and Supplementary Fig. S3B).
GO annotation analysis revealed a strong association between the miRNAs and biological processes, such as the positive regulation of transcription by RNA polymerase II, the regulation of gene expression (both positive and negative), cytokine-mediated signaling, regulation of the apoptotic process, and heart development. Significant GO terms (Benjamini–Hochberg FDR q < 0.001) are shown in Fig. 5A, with detailed miRNA enrichment in biological processes and p-values listed in Supplementary Table S5. GO molecular functions analysis highlighted that the POAF-miRNA signature is highly enriched in transcription binding, DNA-binding transcription factor activity, RNA polymerase II-specific activity, protein binding, RNA polymerase II-cis-regulatory region sequence-specific DNA binding, and protease binding (Fig. 5B). Detailed enrichment in molecular functions, target genes, and p-values are provided in Supplementary Table S6. In terms of cellular components, the POAF-miRNA signature is enriched in the nucleoplasm, nucleus, nuclear chromatin, membrane raft, and cytoplasm (Supplementary Fig. S4), with details in Supplementary Table S7.
POAF-miRNA signature enrichment in gene ontology annotations. (A) Bar plots showing the enrichment of the POAF-miRNA signature in biological processes. (B) Enrichment in molecular functions. The X-axis represents –log10 (p-value) and the Y-axis shows the number of target genes and Gene Ontology term names.
We further identified GO categories specific to cardiovascular diseases. The POAF-miRNA signature is significantly enriched in biological processes including heart development, positive regulation of smooth muscle cell proliferation, striated muscle cell differentiation, cortical actin cytoskeleton organization, and positive regulation of leukocyte adhesion to arterial endothelial cells (Fig. 6A). For molecular functions, the POAF-miRNA signature is enriched in chromatin binding, DNA binding transcription factor activity, enzyme binding, identical protein binding, and protein kinase activity (Fig. 6B). In cellular components, enrichments include caveola, cell–cell junction, COP9 signalosome, cytoplasm, and dopaminergic synapse (Fig. 6C). Detailed information on POAF-miRNA signature enrichment in cardiovascular-related GO categories, target genes, and p-values is available in Supplementary Table S8.
Additionally, the POAF-miRNA signature enriched in Reactome pathways including Interleukin-4 and Interleukin-13 signaling, TNFR1-mediated ceramide production, TNFR1-induced proapoptotic signaling, TNFR1-induced NFkappaB signaling pathway, and Interleukin-10 signaling, as shown in Supplementary Table S9.
POAF-miRNA-gene interaction prediction
We identified key gene targets associated with the POAF-miRNA signature to uncover the genetic network affected by these miRNAs and provide insights into the biological alterations linked to cardiovascular diseases. Using miRWalk, miRNet, miRDB, and miRTarBase, we identified 9,763 gene targets for the POAF-miRNA signature. We focused on gene targets shared by five or more of the POAF-miRNA signature miRNAs (Fig. 7A). This approach led us to identify 50 genes. Next, we used miRNet (2.0)22 to construct a miRNA-gene interaction network with 30 gene targets using the shortest path algorithm (Fig. 7B). We examined the expression of these 30 genes in human dilated and hypertrophic cardiomyopathy. This analysis revealed that these genes are enriched in fibroblasts, cardiomyocyte-I, II, and III, macrophages, and adipocytes (Fig. 7C). Furthermore, we built a miRNA-small molecule interaction network to explore the targets for the POAF-miRNA signature. The POAF-miRNA signature targets various small molecules, as shown in Fig. 7D.
MiRNA-gene target interactions. (A) Upset plot depicting shared gene targets within the POAF-miRNA signature. (B) Network demonstrating connections between POAF-miRNAs (in red rhombus) and target genes (in black circles). (C) Comparative expression levels of POAF-miRNA signature targeted genes in hypertrophic cardiomyopathy. Plot generated from the Broad Institute of MIT and Harvard’s Single Cell Portal. (D) Network plot displaying specific predicted small molecule drugs targeting the miRNA signature, with miRNAs represented in navy blue rhombus and small molecules in burgundy circles.
Influence of medication use on miRNA expression
To investigate the potential influence of medication use on miRNA expression, we analyzed the top five most frequently used medication categories in our cohort—vasodilators/nitrates, statins, beta-blockers, calcium channel blockers, and ACE inhibitors—focusing on their impact on the expression of 10 miRNAs: hsa-let-7a-5p, hsa-miR-1, hsa-miR-100-5p, hsa-miR-106b-5p, hsa-miR-10b-5p, hsa-miR-122-5p, hsa-miR-124-3p, hsa-miR-125b-5p, hsa-miR-126-3p, and hsa-miR-133a-3p. The distribution of these medications is summarized in Table 4.
We compared miRNA expression levels between participants using each medication category and those not using it, using the Mann–Whitney U test due to the small sample size (log2-transformed data assumed for normalization). For beta-blockers, used by 6 participants (3 cases, 3 controls), median expression levels of hsa-miR-1 were 58.8 in users versus 22.1 in non-users (p = 0.15), with similar non-significant trends for other miRNAs (p-values ranging from 0.12 to 0.89). For vasodilators/nitrates, used by 11 participants (6 cases, 5 controls), hsa-miR-126-3p showed a trend toward higher expression in users (median 119.5) compared to non-users (median 29.0, p = 0.09). Statins (10 users: 5 cases, 5 controls) showed no significant differences, with hsa-miR-133a-3p expression at 37.6 in users versus 30.8 in non-users (p = 0.67). Calcium channel blockers (6 users: 2 cases, 4 controls) and ACE inhibitors (6 users: 3 cases, 3 controls) similarly showed no significant differences, with p-values ranging from 0.41 to 0.53 (Supplementary Table S10).
Discussion
Early detection of POAF holds significant promise as it can inform management decisions that alter the natural progression and complication profile of this disease. Traditional parameters, including demographic characteristics such as age, gender, alcohol consumption, tobacco use, and previous medical history, may not reliably predict POAF, especially in studies with small sample sizes. However, molecular information may offer valuable insights.
In this study, we investigated the presence of circulating miRNAs in patients who had undergone CABG by employing microarray and bioinformatics analyses. These techniques allowed us to identify potential biomarkers for predicting POAF. Leveraging machine learning techniques, we selected the top 10 ranked miRNAs out of 84 and evaluated their POAF prediction performance using Random Forest, KNN, XGBoost, and SVM models. The Random Forest and XGBoost models demonstrated superior prediction performance compared to the other methods, with test sensitivities of 0.76 and 0.83, respectively. To further explore the miRNAs differentially expressed between cases and controls, we performed differential expression analysis. We found that four of these upregulated miRNAs—hsa-miR-96-5p, hsa-miR-184, hsa-miR-17-3p, and hsa-miR-200-3p—overlapped with the AF-miRNA signature. While the 13 differentially expressed miRNAs showed significant differences between cases and controls, nine of the 13 miRNAs were not accurately predictive of POAF. Thus, we focused exclusively on the POAF-miRNA signature for further analysis. It is important to note that our preliminary analysis, previously published, identified a different set of miRNAs and primarily involved statistical analysis13. The findings from our current analysis, focusing on miRNA identification, are detailed in this study.
The association between the POAF-miRNA signature and cardiovascular diseases revealed that miRNAs such as hsa-miR-124-3p, hsa-miR-17-5p, hsa-miR-184, hsa-miR-19a-3p, hsa-miR-19b-3p, hsa-miR-423-5p, and hsa-miR-96-5p were significantly associated with various cardiovascular diseases, including acute myocardial infarction, hyperactivity disorder, hypertrophic cardiomyopathy, pulmonary hypertension, and vascular disease. The literature supports these findings. For instance, miR-124-3p promotes cardiac fibroblast activation, while miR-17-5p is associated with acute myocardial ischemia injury and cardiac hypertrophy and serves as a novel biomarker for diagnosing acute myocardial infarction32, while miR-17-5p is associated with acute myocardial ischemia injury and cardiac hypertrophy and serves as a novel biomarker for diagnosing acute myocardial infarction33,34,35. Circulating miR-184 is a potential predictive biomarker for cardiac damage and acts as a biomarker for POAF in patients with valvular heart disease12. Lower expression levels of miR-19a-3p/19b-3p are found in the plasma of heart failure patients, with miR-19b-3p identified as a strong prognostic biomarker for acute heart failure36,37. Increased expression levels of miR-423-5p are linked to heart failure diagnosis38, and miR-96-5p functions as a potential diagnostic biomarker for acute myocardial infarction39.
Further biological relevance of the POAF-miRNAs revealed that these miRNAs target specific KEGG pathways associated with cardiovascular diseases, such as hypertrophic cardiomyopathy (HCM), MAPK signaling pathway, PI3K-Akt signaling pathway, FoxO signaling pathway, and TGF-beta signaling pathway. POAF is a common sequela of HCM, with evidence showing approximately a 20% lifetime risk for the development of POAF in HCM40,41. The HCM pathway maps out the genetic and metabolic interactions involved in the disease. Hundreds of gene mutations in the sarcomere proteins are linked to HCM, increasing the Ca2 + sensitivity of cardiac myofilaments. This heightened sensitivity likely raises ATP utilization, potentially causing an energy imbalance in the heart under stress. Specific miRNAs such as hsa-miR-17-5p and hsa-miR-19a-3p influence key signaling molecules and pathways in HCM, including those involved in cardiac hypertrophy and fibrosis42,43,44. For example, miR-19a-3p and 19b-3p target components of the TGF-β signaling pathway, crucial in the fibrotic response seen in HCM44. KEGG pathway analysis showed that the POAF-miRNAs, including hsa-let-7a-5p, hsa-miR-17-5p, hsa-miR-19a-3p, hsa-miR-124-3p, and hsa-miR-200-3p, were significantly (P < 0.005) involved in the MAPK signaling pathway, PI3K-Akt signaling pathway, and FoxO signaling pathway. These pathways are important in atrial fibrosis in patients with chronic AFib45,46,47,48.
GO annotations provide key insights into the biological processes, cellular components, and molecular functions associated with POAF. POAF-miRNAs play a significant role in AFib by regulating the expression of genes involved in crucial pathways such as heart development and positive regulation of smooth muscle cell proliferation, contributing to the pathogenesis of POAF49,50,51,52. GO annotation analysis also provides insights into the enrichment of POAF-miRNAs in molecular functions, including transcription binding, DNA-binding transcription factor activity, and RNA polymerase II-specific; cellular components such as caveolae, cell–cell junction, COP9 signalosome, and cytoplasm. This comprehensive analysis highlights the importance of the identified POAF-miRNAs in AFib development and cardiovascular disease progression.
Beyond clinical characteristics, lifestyle and metabolic factors such as diet, physical activity, smoking, alcohol consumption, diabetes, hypertension, and obesity may influence circulating miRNA expression and, consequently, the risk of POAF. Several miRNAs implicated in this study, including hsa-miR-214-5p and hsa-miR-590-5p, have been linked to inflammatory responses and immune regulation, which are influenced by these factors53,54. Future studies should explore the interactions between lifestyle modifications, metabolic disorders, and miRNA expression to enhance the predictive accuracy and clinical applicability of miRNA-based POAF risk stratification. Understanding these relationships could also open new avenues for lifestyle-targeted interventions aimed at modulating miRNA signatures to reduce postoperative complications.
Our findings suggest that miRNA-based machine learning models hold promise for POAF risk stratification, offering a noninvasive approach for personalized patient management. These findings have significant clinical implications, as early identification of at-risk patients may allow for targeted preventive strategies, improved perioperative management, and reduced postoperative complications55,56,57. Furthermore, these miRNAs provide mechanistic insights into POAF pathophysiology, highlighting potential avenues for therapeutic intervention58. However, further validation in larger, independent cohorts is crucial to assess the reproducibility and generalizability of these biomarkers. Future studies should aim to incorporate diverse patient populations, evaluate longitudinal miRNA expression changes, and integrate multi-omic approaches to refine predictive accuracy. If successfully validated, miRNA-based screening could be incorporated into preoperative risk assessment protocols, potentially improving clinical decision-making and patient outcomes in CABG surgery.
Limitations to the study
The identified POAF-miRNA signature has the potential to be predictive of POAF in patients undergoing CABG as shown by its significant enrichment in several important pathways that contribute to AFib and other cardiovascular diseases. However, a key limitation of this study is the small sample size, which increases the likelihood of sampling bias and limits the ability to detect small but potentially significant associations. A reduced sample size affects statistical power, increasing the risk of Type II errors (false negatives) and potentially limiting the reproducibility of our findings in larger cohorts. Given the small sample size, there is a risk of overfitting in our machine learning model. To mitigate this, we employed 5-fold cross-validation, regularization techniques, feature selection, and bootstrapping to improve generalizability. Additionally, we used multiple evaluation metrics beyond accuracy to ensure model robustness. However, future validation in larger, independent cohorts is necessary to confirm the reproducibility of our findings and further optimize the predictive model. Additionally, we carefully controlled for potential confounding variables and observed consistent trends with prior studies, suggesting biological relevance. However, we recognize that the confidence intervals for some results may be wider, indicating greater uncertainty in effect estimates. To validate the predictive power of these miRNAs, future studies with larger, independent cohorts will be essential to confirm these preliminary findings and further refine their clinical applicability. The small sample size limits the generalizability and robustness of the findings, necessitating further validation with larger cohorts. Although, we validated out model on an external dataset, validating a serum-derived signature in a dataset with heart tissue miRNA expression data may have affected performance due to the sample-type specificity of miRNA expression. The limited number of cases and controls in the validation dataset restricted statistical power, impacting reliability. The small size of the original training dataset and of the validation dataset also likely led to overfitting and instability. Future directions include increasing the size of the external validation dataset, validating in serum-based miRNA datasets for consistency, and investigating miRNAs with strong individual performance (e.g., hsa-miR-19b-3p, hsa-miR-124-3p, hsa-miR-100-5p) for model refinement. To further assess the robustness of our findings, we conducted a post hoc power analysis based on our observed effect sizes. The results suggest that a larger sample size is needed to achieve optimal statistical power, reinforcing the need for future validation in larger, independent cohorts. While our study provides preliminary evidence for the predictive value of the identified miRNAs, these findings should be interpreted with caution due to the limited statistical power inherent in small-sample studies.
Next, our study cohort predominantly consists of male patients due to the application of strict inclusion and exclusion criteria. While we initially screened 165 patients, only one female participant met all study criteria, leading to a significant gender imbalance. This may limit the generalizability of our findings, as sex-based differences in miRNA expression and POAF pathophysiology have been reported in previous studies. Future studies should aim to include a more balanced male-to-female ratio to assess whether the identified miRNA signatures and their predictive value are consistent across sexes.
Additionally, our study utilized a targeted miRNA PCR array that includes 84 well-characterized circulating miRNAs. While this approach enables high-sensitivity detection of relevant miRNAs, it does not provide a full miRnome-wide assessment. Next-generation sequencing (NGS) would allow for an unbiased discovery of additional miRNAs with potential relevance to POAF. Future large-scale investigations integrating NGS with targeted validation approaches will be essential to refine and expand upon the miRNA signature identified here.
Despite these limitations, our study serves as an exploratory foundation, highlighting the potential role of miRNA-based biomarkers for POAF risk assessment and providing a basis for larger-scale investigations. Furthermore, while the miRNA signatures identified show promise, their predictive power needs to be confirmed through independent validation studies. The study also does not account for potential confounding factors such as medication use, which might influence miRNA expression. Future research should aim to address these limitations by incorporating larger, diverse populations and considering additional variables that may affect miRNA expression.
.
Data availability
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.
References
Everett, T. H. T. & Olgin, J. E. Atrial fibrosis and the mechanisms of atrial fibrillation. Heart Rhythm 4, S24-27. https://doi.org/10.1016/j.hrthm.2006.12.040 (2007).
Auer, J. et al. Risk factors of postoperative atrial fibrillation after cardiac surgery. J. Card. Surg. 20, 425–431. https://doi.org/10.1111/j.1540-8191.2005.2004123.x (2005).
Mathew, J. P. et al. A multicenter risk index for atrial fibrillation after cardiac surgery. JAMA 291, 1720–1729. https://doi.org/10.1001/jama.291.14.1720 (2004).
Fuster, V. et al. 2011 ACCF/AHA/HRS focused updates incorporated into the ACC/AHA/ESC 2006 Guidelines for the management of patients with atrial fibrillation: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines developed in partnership with the European Society of Cardiology and in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. J. Am. Coll. Cardiol. 57, e101-198. https://doi.org/10.1016/j.jacc.2010.09.013 (2011).
Sun, Y. et al. Role of preoperative atorvastatin administration in protection against postoperative atrial fibrillation following conventional coronary artery bypass grafting. Int. Heart J. 52, 7–11. https://doi.org/10.1536/ihj.52.7 (2011).
Attaran, S., Shaw, M., Bond, L., Pullan, M. D. & Fabri, B. M. Atrial fibrillation postcardiac surgery: A common but a morbid complication. Interact Cardiovasc. Thorac. Surg. 12, 772–777. https://doi.org/10.1510/icvts.2010.243782 (2011).
Baker, W. L. & White, C. M. Post-cardiothoracic surgery atrial fibrillation: A review of preventive strategies. Ann. Pharmacother. 41, 587–598. https://doi.org/10.1345/aph.1H594 (2007).
Elahi, M. M., Flatman, S. & Matata, B. M. Tracing the origins of postoperative atrial fibrillation: the concept of oxidative stress-mediated myocardial injury phenomenon. Eur. J. Cardiovasc. Prev. Rehabil. 15, 735–741. https://doi.org/10.1097/HJR.0b013e328317f38a (2008).
Chung, M. K. et al. C-reactive protein elevation in patients with atrial arrhythmias: Inflammatory mechanisms and persistence of atrial fibrillation. Circulation 104, 2886–2891. https://doi.org/10.1161/hc4901.101760 (2001).
Baba, A. & Fu, M. Autoantibodies in atrial fibrillation: Actor, biomaker or bystander?. Autoimmunity 41, 470–472. https://doi.org/10.1080/08916930802031504 (2008).
Andrew, P. & Montenero, A. S. Is there a link between atrial fibrillation and certain bacterial infections?. J. Cardiovasc. Med. (Hagerstown) 8, 990–996. https://doi.org/10.2459/JCM.0b013e32801411e5 (2007).
Sharma Param, P., Sathipati Srinivasulu, Y., Shukla, S., Carter, T. & Soodi, D. Identification of a microrna signature for predicting atrial fibrillation in patients with valvular heart disease. J. Am. Coll. Cardiol. 83, 2185–2185. https://doi.org/10.1016/S0735-1097(24)04175-5 (2024).
Sathipati Srinivasulu, Y. et al. Circulating microrna signature predicts atrial fibrillation in patients following coronary artery bypass grafting. J. Am. Coll. Cardiol. 83, 33–33. https://doi.org/10.1016/S0735-1097(24)02023-0 (2024).
Gilad, S. et al. Serum microRNAs are promising novel biomarkers. PLoS ONE 3, e3148. https://doi.org/10.1371/journal.pone.0003148 (2008).
Barth, A. S. et al. Reprogramming of the human atrial transcriptome in permanent atrial fibrillation: Expression of a ventricular-like genomic signature. Circ. Res. 96, 1022–1029. https://doi.org/10.1161/01.Res.0000165480.82737.33 (2005).
Shen, N. N. et al. Identification of microRNA biomarkers in atrial fibrillation: A protocol for systematic review and bioinformatics analysis. Medicine (Baltimore) 98, e16538. https://doi.org/10.1097/md.0000000000016538 (2019).
Koniari, I. et al. Biomarkers in the clinical management of patients with atrial fibrillation and heart failure. J. Geriatr. Cardiol. 18, 908–951. https://doi.org/10.11909/j.issn.1671-5411.2021.11.010 (2021).
Galenko, O. et al. The role of microRNAs in the development, regulation, and treatment of atrial fibrillation. J. Interv. Card. Electrophysiol. 55, 297–305. https://doi.org/10.1007/s10840-018-0495-z (2019).
Kiliszek, M. et al. Serum microRNA in patients undergoing atrial fibrillation ablation. Sci. Rep. 10, 4424. https://doi.org/10.1038/s41598-020-61322-6 (2020).
Cao, Y. & Cui, L. Identifying the key microRNAs implicated in atrial fibrillation. Anatol. J. Cardiol. 25, 429–436. https://doi.org/10.14744/AnatolJCardiol.2020.41625 (2021).
Cui, C., Zhong, B., Fan, R. & Cui, Q. HMDD v4.0: A database for experimentally supported human microRNA-disease associations. Nucleic Acids Res. 52, D1327–D1332. https://doi.org/10.1093/nar/gkad717 (2024).
Chang, L. & Xia, J. MicroRNA regulatory network analysis using miRNet 2.0. Methods Mol. Biol. 2594, 185–204. https://doi.org/10.1007/978-1-0716-2815-7_14 (2023).
Kanehisa, M., Furumichi, M., Sato, Y., Matsuura, Y. & Ishiguro-Watanabe, M. KEGG: Biological systems database as a model of the real world. Nucleic Acids Res. 53, D672-d677. https://doi.org/10.1093/nar/gkae909 (2025).
Tastsoglou, S. et al. DIANA-miRPath v4.0: Expanding target-based miRNA functional analysis in cell-type and tissue contexts. Nucleic Acids Res. 51, W154–W159. https://doi.org/10.1093/nar/gkad431 (2023).
Sticht, C., De La Torre, C., Parveen, A. & Gretz, N. miRWalk: An online resource for prediction of microRNA binding sites. PLoS ONE 13, e0206239. https://doi.org/10.1371/journal.pone.0206239 (2018).
Chen, Y. & Wang, X. miRDB: An online database for prediction of functional microRNA targets. Nucleic Acids Res. 48, D127-d131. https://doi.org/10.1093/nar/gkz757 (2020).
Huang, H. Y. et al. miRTarBase update 2022: An informative resource for experimentally validated miRNA-target interactions. Nucleic Acids Res. 50, D222-d230. https://doi.org/10.1093/nar/gkab1079 (2022).
Lundberg, S. M. et al. From local explanations to global understanding with explainable AI for trees. Nat. Mach. Intell. 2, 56–67. https://doi.org/10.1038/s42256-019-0138-9 (2020).
Chen, T. & Guestrin, C. 785–794.
Nohara, Y., Matsumoto, K., Soejima, H. & Nakashima, N. Explanation of machine learning models using Shapley additive explanation and application for real data in hospital. Comput. Methods Programs Biomed. 214, 106584 (2022).
Peng, Y., Su, P. & Zhao, L. Long noncoding RNA and messenger RNA profiling in epicardial adipose tissue of patients with new-onset postoperative atrial fibrillation after coronary artery bypass grafting. Eur. J. Med. Res. 29, 134. https://doi.org/10.1186/s40001-024-01721-x (2024).
Zhu, P. et al. MicroRNAs sequencing of plasma exosomes derived from patients with atrial fibrillation: miR-124-3p promotes cardiac fibroblast activation and proliferation by regulating AXIN1. J. Physiol. Biochem. 78, 85–98. https://doi.org/10.1007/s13105-021-00842-9 (2022).
Zhao, L. et al. MiR-17-5p-mediated endoplasmic reticulum stress promotes acute myocardial ischemia injury through targeting Tsg101. Cell Stress Chaperones 26, 77–90. https://doi.org/10.1007/s12192-020-01157-2 (2021).
Xu, X., Su, Y. L., Shi, J. Y., Lu, Q. & Chen, C. MicroRNA-17-5p promotes cardiac hypertrophy by targeting Mfn2 to inhibit autophagy. Cardiovasc. Toxicol. 21, 759–771. https://doi.org/10.1007/s12012-021-09667-w (2021).
Xue, S. et al. Circulating MiR-17-5p, MiR-126-5p and MiR-145-3p are novel biomarkers for diagnosis of acute myocardial infarction. Front Physiol 10, 123. https://doi.org/10.3389/fphys.2019.00123 (2019).
Zou, M. et al. Autophagy inhibition of hsa-miR-19a-3p/19b-3p by targeting TGF-β R II during TGF-β1-induced fibrogenesis in human cardiac fibroblasts. Sci. Rep. 6, 24747. https://doi.org/10.1038/srep24747 (2016).
Su, Y. et al. Circulating miR-19b-3p as a novel prognostic biomarker for acute heart failure. J. Am. Heart Assoc. 10, e022304. https://doi.org/10.1161/JAHA.121.022304 (2021).
Tijsen, A. J. et al. MiR423-5p as a circulating biomarker for heart failure. Circ. Res. 106, 1035–1039. https://doi.org/10.1161/CIRCRESAHA.110.218297 (2010).
Ding, H., Chen, W. & Chen, X. Serum miR-96-5p is a novel and non-invasive marker of acute myocardial infarction associated with coronary artery disease. Bioengineered 13, 3930–3943. https://doi.org/10.1080/21655979.2022.2031392 (2022).
Siontis, K. C. et al. Atrial fibrillation in hypertrophic cardiomyopathy: Prevalence, clinical correlations, and mortality in a large high-risk population. J. Am. Heart Assoc. 3, e001002. https://doi.org/10.1161/JAHA.114.001002 (2014).
Olivotto, I. et al. Impact of atrial fibrillation on the clinical course of hypertrophic cardiomyopathy. Circulation 104, 2517–2524 (2001).
Sun, Y., Xiao, Z., Chen, Y., Xu, D. & Chen, S. Susceptibility modules and genes in hypertrophic cardiomyopathy by WGCNA and ceRNA network analysis. Front. Cell Dev. Biol. 9, 822465 (2022).
Shi, H. et al. Systematic identification and analysis of dysregulated mi RNA and transcription factor feed-forward loops in hypertrophic cardiomyopathy. J. Cell Mol. Med. 23, 306–316 (2019).
Zou, M. et al. Autophagy inhibition of hsa-miR-19a-3p/19b-3p by targeting TGF-β R II during TGF-β1-induced fibrogenesis in human cardiac fibroblasts. Sci. Rep. 6, 24747 (2016).
Zhang, D. et al. Role of the MAPKs/TGF-β1/TRAF6 signaling pathway in postoperative atrial fibrillation. PLoS ONE 12, e0173759 (2017).
Zhang, D. et al. Role of the MAPKs/TGF-β1/TRAF6 signaling pathway in atrial fibrosis of patients with chronic atrial fibrillation and rheumatic mitral valve disease. Cardiology 129, 216–223 (2014).
McMullen, J. R. et al. Ibrutinib increases the risk of atrial fibrillation, potentially through inhibition of cardiac PI3K-Akt signaling. Blood J. Am. Soc. Hematol. 124, 3829–3830 (2014).
Yu, W., Chen, C. & Cheng, J. The role and molecular mechanism of FoxO1 in mediating cardiac hypertrophy. ESC Heart Fail. 7, 3497–3504 (2020).
Wang, D. et al. Long non-coding RNA MALAT1 sponges miR-124-3p. 1/KLF5 to promote pulmonary vascular remodeling and cell cycle progression of pulmonary artery hypertension. Int. J. Mol. Med. 44, 871–884 (2019).
Sharma, V. et al. Integrative experimental validation of concomitant miRNAs and transcription factors with differentially expressed genes in acute myocardial infarction. Eur. J. Pharmacol. 971, 176540 (2024).
Vatan, M. B. et al. Altered plasma microRNA expression in patients with mitral chordae tendineae rupture. J. Heart Valve Dis 25, 580–588 (2016).
Su, Y. et al. Circulating miR-19b-3p as a novel prognostic biomarker for acute heart failure. J. Am. Heart Assoc. 10, e022304 (2021).
Włodarski, A. et al. The Role of microRNAs in metabolic syndrome-related oxidative stress. Int. J. Mol. Sci. https://doi.org/10.3390/ijms21186902 (2020).
Zhang, J., Li, A., Gu, R., Tong, Y. & Cheng, J. Role and regulatory mechanism of microRNA mediated neuroinflammation in neuronal system diseases. Front. Immunol. 14, 1238930. https://doi.org/10.3389/fimmu.2023.1238930 (2023).
Panico, A. et al. The influence of lifestyle factors on miRNA expression and signal pathways: A review. Epigenomics 13, 145–164 (2021).
Pointner, A. et al. Lifestyle-driven variations in Nutrimiromic MicroRNA expression patterns across and beyond genders. Life 14, 390 (2024).
Włodarski, A. et al. The role of microRNAs in metabolic syndrome-related oxidative stress. Int. J. Mol. Sci. 21, 6902 (2020).
Wang, Z. Y. et al. Advances in point-of-care testing of micrornas based on portable instruments and visual detection. Biosensors (Basel) https://doi.org/10.3390/bios13070747 (2023).
Acknowledgements
This work was supported in part by the Marshfield Clinic Research Institute, Marshfield, WI. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
S.Y.S and P.S. supervised and carried out the detail study. S.Y.S, T.C, D.S, N.S, S.K.S, J.P, G.I, J.B and P.S participated in data analysis, manuscript preparation and discussed the results. All authors have read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Yerukala Sathipati, S., Carter, T., Soodi, D. et al. MicroRNA signature predicts post operative atrial fibrillation after coronary artery bypass grafting. Sci Rep 15, 18658 (2025). https://doi.org/10.1038/s41598-025-03042-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-03042-3